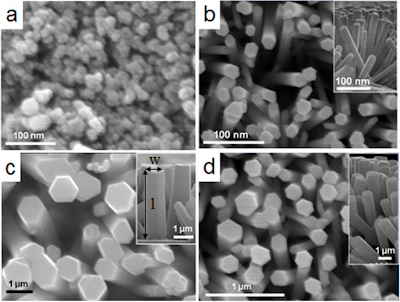
Solvent bar microextraction of emerging pollutants from drain water samples
The efficiency of a given microextraction technique depends on both thermodynamic and kinetic aspects. The distribution constant defines the maximum extractable analyte whereas the kinetic establishes the rate at which this distribution takes place. Among the kinetic factors, the efficient diffusion of the target analytes from the bulk sample solution to the acceptor phase is a key aspect. This diffusion can be easily enhanced by an efficient stirring of the sample or the acceptor phase during the extraction. Solvent bar microextraction (SBME), which was firstly presented by Jiang and Lee in 2004, (1) enhances the diffusion of the analytes through an efficient stirring of the acceptor phase. SBME uses a solvent immobilized in the lumen and pores of a polypropylene hollow fiber as extracting phase. Both ends of the hollow fiber are sealed and the resulting solvent bar is introduced in the sample where it moves free and randomly. After the extraction, the solvent bar is recovered a